Significantly good! What if the mask manufacturing enterprise is not listed on the customs whitelist
- Author:MIKEY
- Source:Sunny Worldwide Logistics
- Release Date:2020-04-29
Yesterday, Sohang.com, according to official announcements issued by the Ministry of Commerce, the State Administration of Market Supervision, the US FDA and other authoritative agencies, exclusively released the "blacklist" of export enterprises for masks and other anti-epidemic materials! A list of 2,235 whitelisted companies is attached!
With a single name, domestic mask production, export companies, logistics and customs companies immediately acted to quickly check whether various customers entered this whitelist of so-called "green channels for customs clearance"!
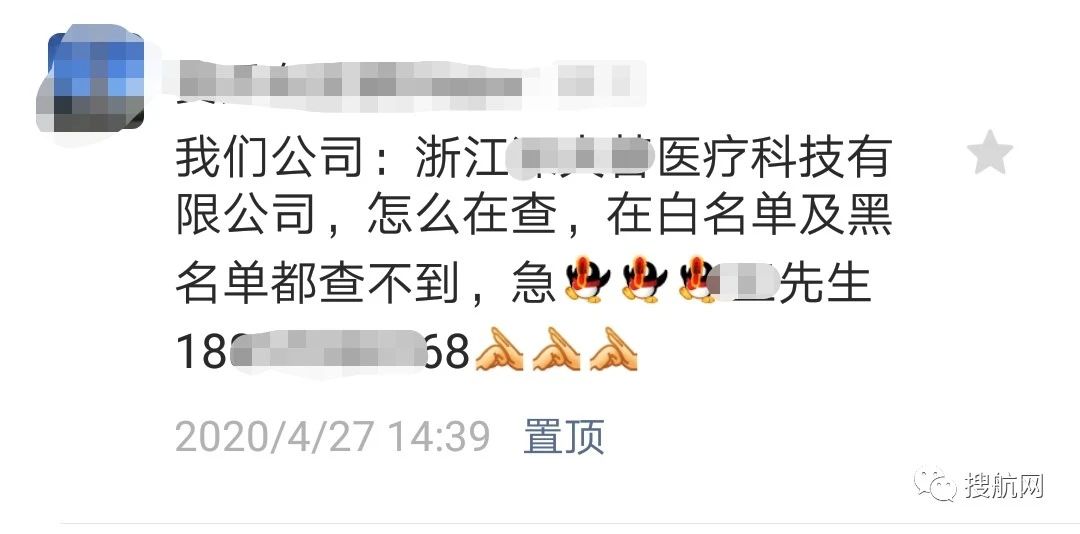
▲ Companies that could not be found are very anxious!
However, at present, the mask companies that have entered the first batch of official whitelists are after all a minority. Are there no opportunities for export companies that have not entered the whitelist?
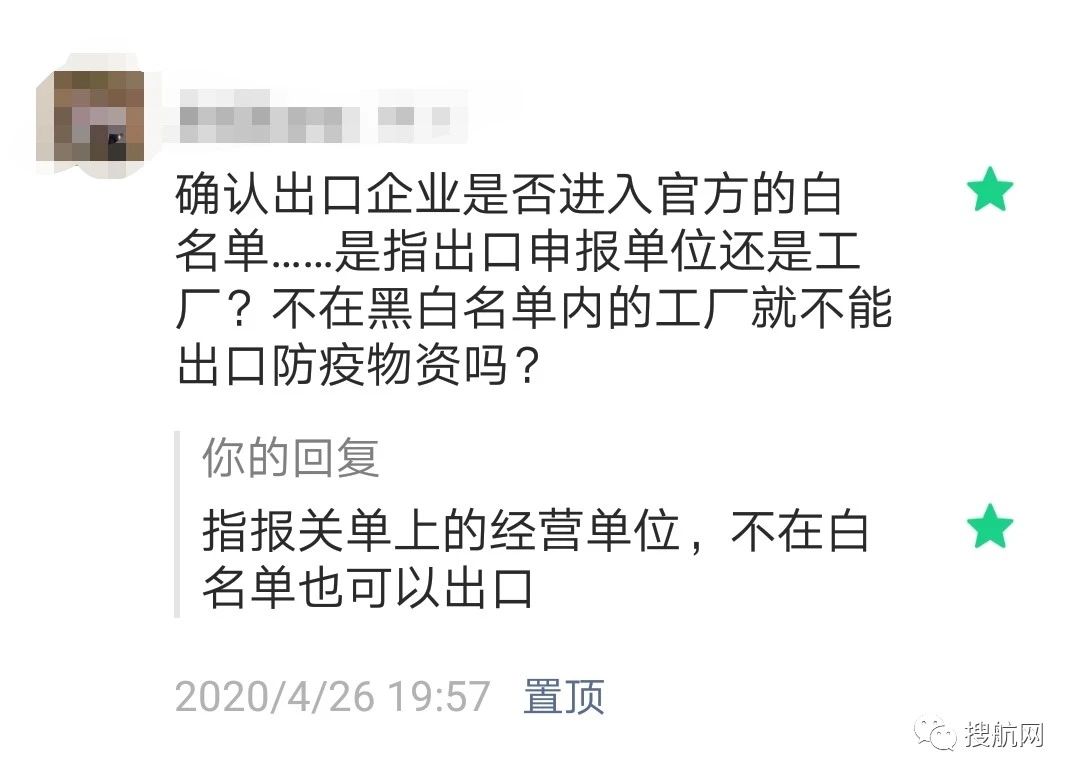
▲ If you do not enter the white list, it does not mean that you cannot export!
So, for the other nearly 100,000 mask production enterprises, the most concerned question is: how to apply for the white list?
Yesterday (April 26), the Ministry of Commerce issued an emergency notice announcing that epidemic prevention material production enterprises can apply to the local Commerce Bureau to join the Ministry of Commerce white list, but they need to submit the following relevant forms and supporting materials in accordance with regulations.
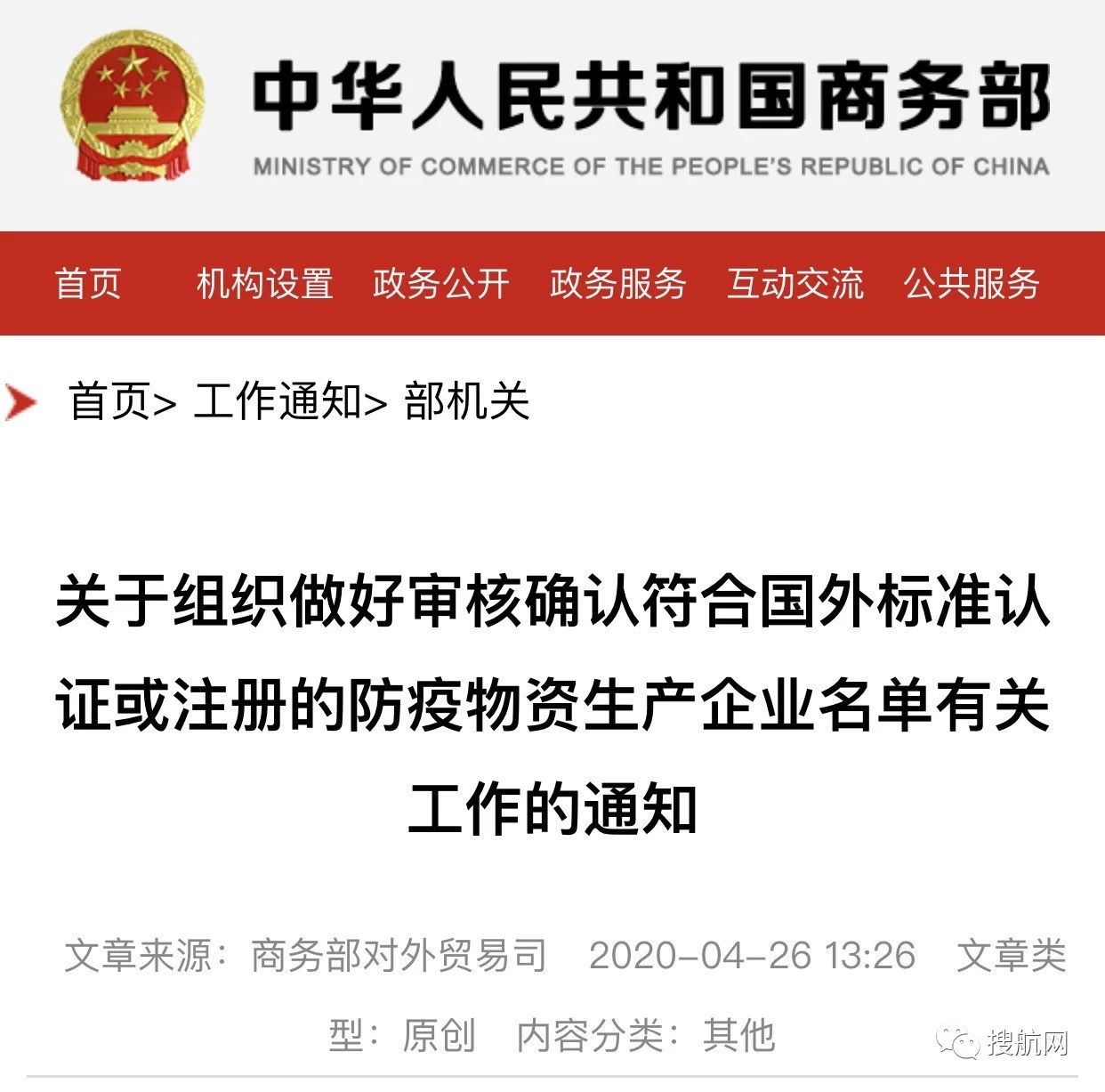
Notice
Notice on organizing the work of reviewing and confirming the list of anti-epidemic material production enterprises that meet the certification or registration of foreign standards
Commercial departments of provinces, autonomous regions, municipalities directly under the Central Government and Xinjiang Production and Construction Corps:
In order to implement the "Announcement of the State Administration of Market Supervision and Administration of the General Administration of Customs of the Ministry of Commerce on Further Strengthening the Quality Supervision of the Export of Anti-epidemic Materials" (No. 12 of 2020), we now review and confirm the production of anti-epidemic materials that conform to the certification or registration of foreign standards The relevant work notice of the enterprise list is as follows:
All local competent commercial departments are invited to organize local epidemic prevention material production enterprises to voluntarily fill in the relevant forms and submit relevant certification materials:
Non-medical mask manufacturers fill in Annex 1,
Manufacturers of 5 types of medical supplies such as medical masks fill in Annex 2
After the preliminary review by the local commercial competent department and the relevant member units of the local medical materials commercial export working mechanism, the summary form (including the electronic version) shall be uniformly submitted to the national medical materials commercial export work in the name of the working mechanism office (local commercial competent authority seal). Mechanism Office (Foreign Trade Department of the Ministry of Commerce), also copied to the China Chamber of Commerce for Import and Export of Medicines and Health Products.
In principle, submit once a week, and the deadline is 17:00 every Wednesday.
annex:
1. List of non-medical mask manufacturing enterprises that have obtained foreign standard certification or registration.xlsx
2. List of medical material manufacturing enterprises that have obtained foreign standard certification or registration.xlsx
National Medical Materials Commercial Export Working Mechanism Office
April 26, 2020
attachment1:
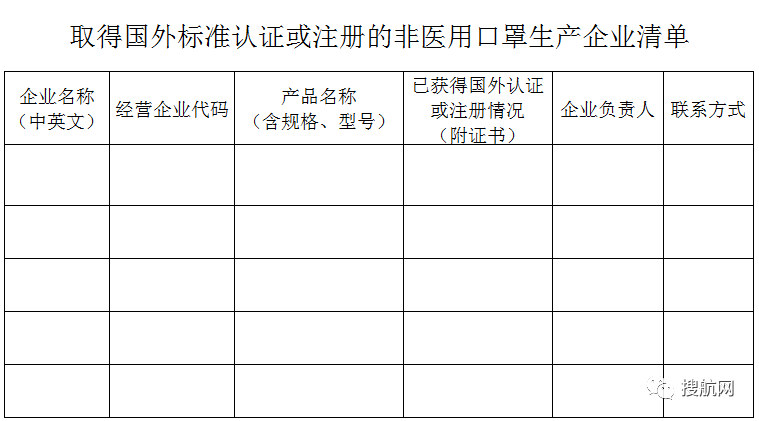
Completion Instructions:
1. The scope of application is limited to non-medical masks that have obtained foreign certification or registration;
2. Please fill in by product, each product line;
3. Be sure to fill in the Chinese and English names in the "Company Name" column. Please fill in multiple products of the same company in sequence. The company name column does not merge cells;
4. "Product name" please specify the specific names, specifications, models, etc. in Chinese and English;
5. Please be sure to submit the scanned version of the foreign certification key materials filled in this form, including the certification certificate, test report, etc., and name the file with the certificate name;
6. Relevant documents should be placed in a folder, the folder is named after the Chinese name of the enterprise, and the relevant qualification file is named: serial number + abbreviation of the Chinese name of the enterprise + product name + certificate or test report name.
Attachment 2:
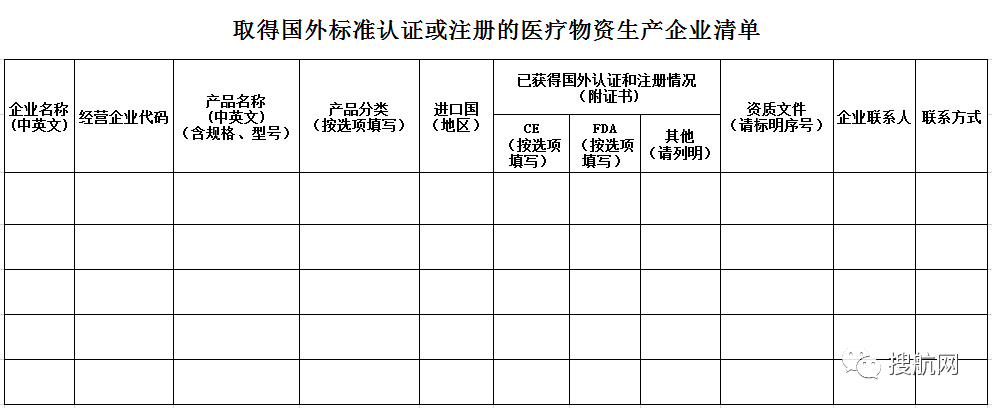
Note:
1. Only for companies that have signed contracts after April 1st (inclusive) and whose export products have not obtained the medical device product registration certificate of China;
2. Please report that the company must provide a scanned copy of the foreign standard certification materials that have been filled out, and the relevant proof that the foreign party accepts the standards and quality of the purchased product.
Completion Instructions:
1. The scope of the report is limited to five major types of medical materials, namely new crest virus detection reagents, ventilator, medical protective clothing, medical mask, infrared thermometer;
2. Please fill in by product, each product line;
3. Be sure to fill in the Chinese and English names in the "Company Name" column. Please fill in multiple products of the same company in sequence. The company name column does not merge cells;
4. "Product name" please list the specific names, specifications, models, etc. in Chinese and English, and indicate aseptic or non-sterile;
5. The same product is exported to different countries (regions), please fill in the "Importing Country (Region)" cell, without the need for a line for each country;
6. Please be sure to submit the foreign certification and registration documents that have been filled out in this form, and list them in the same cell in the "Qualification Document" column by serial number;
7. The "qualification documents" filled in this form should be placed in a folder, the folder is named after the Chinese name of the company, and the relevant qualification files are named: serial number + abbreviation of the Chinese name of the enterprise + product name + certificate or test The name and serial number of the report must be the same as the serial number in the "Qualification Document" column of the table.
8. Others: In principle, the export of products should meet the standards of the importing country. If they are not registered in the importing country, please provide the relevant certification documents of the import license authorized by the other country and stipulate in the contract.
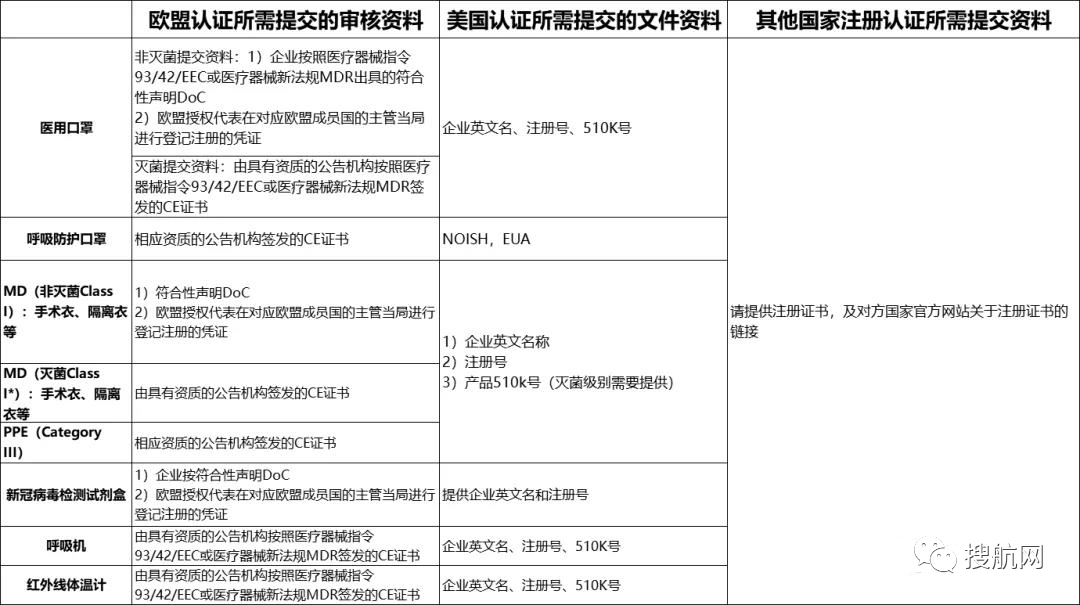
special reminder:
Main emergency materials certification and audit information, please refer to this and submit complete information according to product categories, so as to speed up the audit. It is recommended that one type of product has one folder, and the number of materials provided in each folder is numbered according to the country of registration.
The original link of the official announcement of the Ministry of Commerce:
http://www.mofcom.gov.cn/article/b/e/202004/20200402959046.shtml
(The article is compiled from the Ministry of Commerce and Customs Primary 2)
With a single name, domestic mask production, export companies, logistics and customs companies immediately acted to quickly check whether various customers entered this whitelist of so-called "green channels for customs clearance"!

▲ Companies that could not be found are very anxious!
However, at present, the mask companies that have entered the first batch of official whitelists are after all a minority. Are there no opportunities for export companies that have not entered the whitelist?

▲ If you do not enter the white list, it does not mean that you cannot export!
So, for the other nearly 100,000 mask production enterprises, the most concerned question is: how to apply for the white list?
Yesterday (April 26), the Ministry of Commerce issued an emergency notice announcing that epidemic prevention material production enterprises can apply to the local Commerce Bureau to join the Ministry of Commerce white list, but they need to submit the following relevant forms and supporting materials in accordance with regulations.

Notice
Notice on organizing the work of reviewing and confirming the list of anti-epidemic material production enterprises that meet the certification or registration of foreign standards
Commercial departments of provinces, autonomous regions, municipalities directly under the Central Government and Xinjiang Production and Construction Corps:
In order to implement the "Announcement of the State Administration of Market Supervision and Administration of the General Administration of Customs of the Ministry of Commerce on Further Strengthening the Quality Supervision of the Export of Anti-epidemic Materials" (No. 12 of 2020), we now review and confirm the production of anti-epidemic materials that conform to the certification or registration of foreign standards The relevant work notice of the enterprise list is as follows:
All local competent commercial departments are invited to organize local epidemic prevention material production enterprises to voluntarily fill in the relevant forms and submit relevant certification materials:
Non-medical mask manufacturers fill in Annex 1,
Manufacturers of 5 types of medical supplies such as medical masks fill in Annex 2
After the preliminary review by the local commercial competent department and the relevant member units of the local medical materials commercial export working mechanism, the summary form (including the electronic version) shall be uniformly submitted to the national medical materials commercial export work in the name of the working mechanism office (local commercial competent authority seal). Mechanism Office (Foreign Trade Department of the Ministry of Commerce), also copied to the China Chamber of Commerce for Import and Export of Medicines and Health Products.
In principle, submit once a week, and the deadline is 17:00 every Wednesday.
annex:
1. List of non-medical mask manufacturing enterprises that have obtained foreign standard certification or registration.xlsx
2. List of medical material manufacturing enterprises that have obtained foreign standard certification or registration.xlsx
National Medical Materials Commercial Export Working Mechanism Office
April 26, 2020
attachment1:

Completion Instructions:
1. The scope of application is limited to non-medical masks that have obtained foreign certification or registration;
2. Please fill in by product, each product line;
3. Be sure to fill in the Chinese and English names in the "Company Name" column. Please fill in multiple products of the same company in sequence. The company name column does not merge cells;
4. "Product name" please specify the specific names, specifications, models, etc. in Chinese and English;
5. Please be sure to submit the scanned version of the foreign certification key materials filled in this form, including the certification certificate, test report, etc., and name the file with the certificate name;
6. Relevant documents should be placed in a folder, the folder is named after the Chinese name of the enterprise, and the relevant qualification file is named: serial number + abbreviation of the Chinese name of the enterprise + product name + certificate or test report name.
Attachment 2:

Note:
1. Only for companies that have signed contracts after April 1st (inclusive) and whose export products have not obtained the medical device product registration certificate of China;
2. Please report that the company must provide a scanned copy of the foreign standard certification materials that have been filled out, and the relevant proof that the foreign party accepts the standards and quality of the purchased product.
Completion Instructions:
1. The scope of the report is limited to five major types of medical materials, namely new crest virus detection reagents, ventilator, medical protective clothing, medical mask, infrared thermometer;
2. Please fill in by product, each product line;
3. Be sure to fill in the Chinese and English names in the "Company Name" column. Please fill in multiple products of the same company in sequence. The company name column does not merge cells;
4. "Product name" please list the specific names, specifications, models, etc. in Chinese and English, and indicate aseptic or non-sterile;
5. The same product is exported to different countries (regions), please fill in the "Importing Country (Region)" cell, without the need for a line for each country;
6. Please be sure to submit the foreign certification and registration documents that have been filled out in this form, and list them in the same cell in the "Qualification Document" column by serial number;
7. The "qualification documents" filled in this form should be placed in a folder, the folder is named after the Chinese name of the company, and the relevant qualification files are named: serial number + abbreviation of the Chinese name of the enterprise + product name + certificate or test The name and serial number of the report must be the same as the serial number in the "Qualification Document" column of the table.
8. Others: In principle, the export of products should meet the standards of the importing country. If they are not registered in the importing country, please provide the relevant certification documents of the import license authorized by the other country and stipulate in the contract.

special reminder:
Main emergency materials certification and audit information, please refer to this and submit complete information according to product categories, so as to speed up the audit. It is recommended that one type of product has one folder, and the number of materials provided in each folder is numbered according to the country of registration.
The original link of the official announcement of the Ministry of Commerce:
http://www.mofcom.gov.cn/article/b/e/202004/20200402959046.shtml
(The article is compiled from the Ministry of Commerce and Customs Primary 2)
