Masks and other medical supplies are exported to Australia, this rule must be known!
- Author:Anma
- Release Date:2020-03-31
According to the latest news from sunny worldwide Logistics, for Australians exporting medical supplies such as masks, the Australian authorities require the following:

Australian imported masks, protective clothing, goggles, gloves, if they are packaged, and the medical instructions on the product description are subject to TGA supervision, they need to be registered with the TGA before they can be imported. They need to pay an annual fee. CE registered non-medical disposable masks are not required If there is no antibacterial and bacteriostatic in the TGA registered hand sanitizer packaging and instructions, the scope of cosmetics that is not used in medical institutions needs to be registered with NICNAS.
https://www.nicnas.gov.au/cosmetics-and-soaps/hand-sanitisers
If it is medical, it is regulated by TGA.
Conclusion: Imports of Australian products require information on goods such as masks approved by TGA.
I believe that some foreign trade partners may have the following doubts:
1) What is TGA?
TGA, the Australian Medicines Agency, is the federal drug authority under the Australian Department of Health.
Compared with HACCP, FDA and other auditing systems, TGA registration includes evaluation of new drugs, formulation of standards, determination of testing methods, implementation of testing, issue of drug manufacturing licenses, supervision of drug production processes, sampling of drug markets, inspection of drug manufacturing plants, inspection of drug production A series of management work of recording and handling complaints, such as review and supervision, is recognized as one of the world's most stringent drug management systems and the most difficult market access product review system.
Products that have passed TGA certification meet the applicable standards, which means that they are recognized by the Australian government on the quality system and production environment. TGA certification is the GMP certification of the Australian government and enjoys a high reputation internationally, which means that To this permit, it has also been recognized by more than 20 countries including Britain, France, Germany, and Canada.
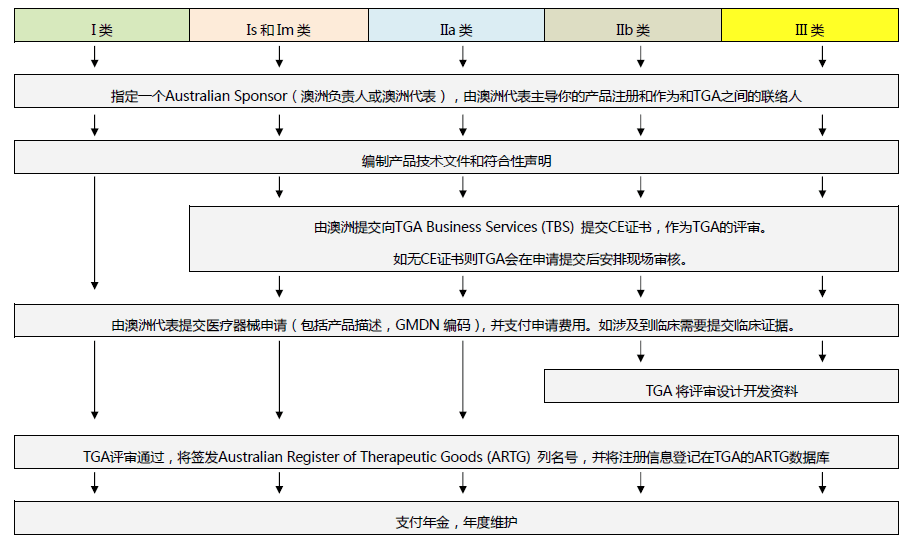
2) TGA related information about medical supplies such as masks?
TGA divides them into two categories from this website
https://www.tga.gov.au/media-release/covid-19-advice-surgical-masks-and-gowns
Statements and claims about masks and protective clothing will determine how TGA regulates these products under the Therapeutic Supplies Act 1989 (the Act).
1. The TGA designs non-sterile masks and gowns under the Act as safety or protective clothing for home or entertainment or professional use.
2. Masks or protective suits proposed or claimed for therapeutic use, such as substances marked for surgical use, or substances used to reduce or prevent the spread of disease or microorganisms such as bacteria or viruses, shall conform to medically defined devices and be subject to the Act TGA specifications.
If these masks or protective clothing are provided in a non-sterile form, they are likely to be regulated as a low risk, Class I medical device.
This includes masks, gloves, masks, goggles, and protective clothing designed to protect the wearer from injury or the spread of infection or disease.
Surgical masks are fluid-resistant, disposable and loose-fitting protective devices that can form a physical barrier between the wearer's nose and mouth and the surrounding environment.
If your customers bring in a mask designed to prevent the spread of viruses, etc. or claim to have it, they must be regulated to prevent import.

Australian imported masks, protective clothing, goggles, gloves, if they are packaged, and the medical instructions on the product description are subject to TGA supervision, they need to be registered with the TGA before they can be imported. They need to pay an annual fee. CE registered non-medical disposable masks are not required If there is no antibacterial and bacteriostatic in the TGA registered hand sanitizer packaging and instructions, the scope of cosmetics that is not used in medical institutions needs to be registered with NICNAS.
https://www.nicnas.gov.au/cosmetics-and-soaps/hand-sanitisers
If it is medical, it is regulated by TGA.
Conclusion: Imports of Australian products require information on goods such as masks approved by TGA.
I believe that some foreign trade partners may have the following doubts:
1) What is TGA?
TGA, the Australian Medicines Agency, is the federal drug authority under the Australian Department of Health.
Compared with HACCP, FDA and other auditing systems, TGA registration includes evaluation of new drugs, formulation of standards, determination of testing methods, implementation of testing, issue of drug manufacturing licenses, supervision of drug production processes, sampling of drug markets, inspection of drug manufacturing plants, inspection of drug production A series of management work of recording and handling complaints, such as review and supervision, is recognized as one of the world's most stringent drug management systems and the most difficult market access product review system.
Products that have passed TGA certification meet the applicable standards, which means that they are recognized by the Australian government on the quality system and production environment. TGA certification is the GMP certification of the Australian government and enjoys a high reputation internationally, which means that To this permit, it has also been recognized by more than 20 countries including Britain, France, Germany, and Canada.

2) TGA related information about medical supplies such as masks?
TGA divides them into two categories from this website
https://www.tga.gov.au/media-release/covid-19-advice-surgical-masks-and-gowns
Statements and claims about masks and protective clothing will determine how TGA regulates these products under the Therapeutic Supplies Act 1989 (the Act).
1. The TGA designs non-sterile masks and gowns under the Act as safety or protective clothing for home or entertainment or professional use.
2. Masks or protective suits proposed or claimed for therapeutic use, such as substances marked for surgical use, or substances used to reduce or prevent the spread of disease or microorganisms such as bacteria or viruses, shall conform to medically defined devices and be subject to the Act TGA specifications.
If these masks or protective clothing are provided in a non-sterile form, they are likely to be regulated as a low risk, Class I medical device.
This includes masks, gloves, masks, goggles, and protective clothing designed to protect the wearer from injury or the spread of infection or disease.
Surgical masks are fluid-resistant, disposable and loose-fitting protective devices that can form a physical barrier between the wearer's nose and mouth and the surrounding environment.
If your customers bring in a mask designed to prevent the spread of viruses, etc. or claim to have it, they must be regulated to prevent import.
